VDx Veterinary Diagnostics
Accurate answers. Expert guidance.
VDx continually sets the standard for diagnostic excellence, service reliability and client satisfaction in Northern California and the Western United States.
Our Goal
Our goal at VDx is to give you accurate and reliable answers, even on tough cases, with a focus on customer service and consistent rapid turn-around, so you can deliver the highest level of care to your patients.
We are experts in veterinary pathology: Biopsy, Cytology and Advanced Cancer Diagnostics. The staff and pathologists of VDx process more than 30,000 laboratory specimens each year.
Veterinary Diagnostics service forms and resources
Why choose VDx?
Rapid Turn Around Times
Quick turnaround times without the loss of quality and accuracy. Cytology results will be out day of sample receipt and biopsy results will be out the next business day from sample receipt, excluding samples needing additional fixation or decalcification.
Exceptional Customer Service
We pride ourselves on our excellent customer service – we always have a live person speak with you! We can be reached via email or phone to order additional tests, supplies, provide report status and answer questions.
On-Site Veterinary Pathologists You Can Talk To
Our pathologists work on site with your samples, so we are always available to consult with you on tough cases and to lay our hands on tough samples. Call our office to speak with a pathologist about a report.
Mail-In Option
We have clients from all over the nation, so we encourage you to send your samples to us. To offset shipping fees, we offer a credit, per patient, for samples shipped via FedEx/UPS.
Convenient Courier Service
Experienced
VDx has been servicing the diagnostic needs of Northern California for 20+ years with a team of 11 trusted and experienced ACVP board-certified pathologists. They are trusted by Veterinary Specialists all over the U.S.
Biopsy
Our team of ACVP board-certified pathologists have expertise in:
- Internal medicine
- Oncology
- Dermatopathology
- Oral pathology
We also offer advanced diagnostic capabilities including immunohistochemistry and PARR analysis, to help diagnose your tough cases!
Questions on sample submission, preparation, or testing?
VDx customer service and the clinical pathology team are available during business hours to assist you. Contact us.
Results on most cases are available the next business day of sample receipt at the laboratory (Monday-Friday, except holidays).
Samples requiring additional fixation or decalcification will require longer to process.
Cytology
Our team of ACVP board-certified pathologists have expertise in:
- Internal medicine
- Oncology
- Dermatopathology
- Oral pathology
We also offer advanced diagnostic capabilities including immunocytochemistry, PARR analysis, and flow cytometry to help diagnose your tough cases!
Cytology Testing offered for:
- FNA/Impression Smears
- Fluid Analysis
- Bone Marrow Analysis
- CSF
- Alkaline Phosphatase ICC also available!
Questions on sample submission, preparation, or testing?
VDx customer service and the clinical pathology team are available during business hours to assist you. Contact us
Cytology results are available the same business day of sample receipt at the laboratory (Monday-Friday, except holidays).
Cytology STAT results are available Monday-Friday (except holidays) with no additional charge; results are expected by 11 am
Cytology Saturday STAT results are available by noon for an additional charge.
PARR Testing
Unlocking complex diagnostics
Is this cat’s chronic lymphocytic enteritis really chronic IBD or is this early small cell lymphoma? Is this dog’s splenic nodule or enlarged lymph node benign or really indolent lymphoma? VDx is here to help! VDx is currently the only private, non-academic laboratory in the U.S. to offer PARR testing on formalin-fixed tissue in addition to cytology samples.
Why choose VDx Veterinary Diagnostics for PARR testing?
PARR analysis can be run on biopsy or cytology specimens, including those already evaluated at other labs. PARR testing at VDx is rendered with a full evaluation by a pathologist, which includes review of the biopsy/cytology specimen and any IHC/ICC or flow cytometry data.
Turnaround time is 7-10 working days from sample receipt at the laboratory/test order date.
Getting Started
Sending a sample to VDx Veterinary Diagnostics is easy. Please call 1-530-753-4285 or email info@vdxpathology.com and a laboratory assistant will be more than happy to provide you with submission information and pricing.
PARR Testing Frequently Asked Questions
How to submit PARR
What is PARR / Clonality PCR?
What samples are suitable for PARR?
When is PARR Testing appropriate?
How is PARR different from immunohistochemistry and immunocytochemistry?
How is PARR different from flow cytometry?
Is all PARR testing equivalent?
What are the sensitivity and specificity of PARR?
Are there false negatives and false positives with PARR?
What is PARR / Clonality PCR?
PCR for Antigen Receptor Rearrangement (aka PARR or Clonality PCR) is a tool that has greatly increased the sensitivity of diagnosing lymphoma in difficult cases, helping diagnose lymphoma earlier and with less invasive sampling.
This technique is based on the fact that during maturation, lymphocytes undergo a series of genetic alterations (VDJ rearrangements) which are unique from cell to cell, and ultimately culminate in almost unlimited variety among the antigen receptors on B and T lymphocytes. By using PCR primers to amplify the area encoding diversity of the immunoglobulin heavy chain (IgH) of B cells and the gamma subunit of the T cell receptor of T cells (TCRy), DNA from clinical specimens can be analyzed to determine whether the lymphocytes in a specimen share identical antigen receptors (ie are “clonal”), or whether they are genetically different (ie “polyclonal”). As suggested by the clonal theory of cancer, a clonal expansion is very highly suggestive of neoplasia.
When is PARR Testing appropriate?
PARR testing is especially useful when the results of biopsy or cytology evaluation are inconclusive or are suggestive, but insufficient for a definitive diagnosis, of lymphoma.
PARR should be conducted as part of a stepwise process, which begins with clinical assessment (presentation, history, and other lab work) along with microscopic evaluation of tissue (cytology or biopsy) and in some cases immunohistochemistry/cytochemistry.
It can be tempting to view PARR as a shortcut to the diagnosis of lymphoma. However, when steps are omitted (eg biopsy or cytology, immunohistochemistry), misdiagnosis or errors in interpretation may sometimes occur. To prevent this, PARR evaluation at VDx always includes a morphologic review by a pathologist.
What samples are suitable for PARR?
VDx can perform PARR analysis on the following types of canine and feline specimens:
- Cytology samples: including air-dried FNAs and fluid or blood smears (stained or unstained).
- Biopsy samples: including formalin-fixed tissue, as well as paraffin-embedded tissue from unstained
recut slides or paraffin blocks.
We can also analyze biopsy and cytology samples processed at other labs. Biopsy and cytology samples not initially evaluated at VDx will also be evaluated by a VDx pathologist in conjunction with PARR evaluation. To submit samples previously evaluated elsewhere, please request the following from the originating lab:
- Cytology: All stained and unstained cytology slides. VDx will review and choose the best slides.
- Biopsy: Paraffin block(s) (preferred), or at least ten unstained recuts on positively charged slides.
Note: Material sent from other labs will be consumed during testing, in whole or in part, and cannot be returned to the originating lab/party.
How to submit PARR?
Biopsy
We can run PARR on formalin-fixed tissue, including biopsy slides and FFPE tissue blocks.
To submit samples for PARR on a case where a biopsy has already been run, we recommend either sending an FFPE tissue block or at least 10 unstained slides (on positively charged glass) of FFPE tissue. Please also provide an H&E stained slide for each block on the case.
If a biopsy has not been run, tissue can be either sent in a jar of formalin or fresh (we will then transfer to formalin upon specimen arrival).
Please include a VDx submittal form and select the PARR option that fits best – PARR Testing if a biopsy was previously done on the tissue, or PARR Profile if a biopsy is also needed to be run.
If shipping to VDx, during the warmer months, we recommend including an ice pack wrapped in a towel(s) to keep paraffin blocks from melting.
Cytology
Please note: Cytology submissions may only be processed as a PARR Profile and will include a full cytology evaluation as well as clonality results.
If possible, please submit 10-15 unstained slides. Stained slides are okay to send as well, but it is a good idea to include unstained ones in case immunocytochemistry is needed/recommended.
Please submit slides in a secure pap container. If shipping, please include plenty of packing material to cushion slides in the package.
Please include a VDx submittal form and select the PARR Profile.
How is PARR different from immunohistochemistry and immunocytochemistry?
Immunohistochemistry (aka IHC, for biopsies) and immunocytochemistry (aka ICC, for cytologies) are techniques utilizing antibodies to specific cellular components (such as parts of the B and T cell antigen receptors) to determine the phenotype or lineage of cells in a specimen. IHC/ICC allows determination of the types of cells that are present but cannot directly differentiate neoplasia vs. reactive change (ie it cannot determine whether a population of T or B cells is genetically identical).
However, IHC/ICC is used as an adjunct to the evaluation of cellular morphology and assessment of tissue architecture (ie cytology and biopsy evaluation) and to assist in the interpretation of PARR results. In some difficult lymphoma diagnoses, IHC/ICC must also be performed and interpreted in conjunction with PARR to arrive at an accurate diagnosis. The pathologist will advise if this is the case. Omitting IHC/ICC can lead to an increased risk of misinterpretation of PARR results.
How is PARR different from flow cytometry?
Flow cytometry (or “flow”), is similar to IHC, but performed on fluid samples or cell suspensions. Like IHC/ICC, flow also uses antibodies to detect various cell surface markers, but because flow is performed on unfixed cells, a greater array of cell markers can be evaluated, allowing more fine differentiation among various classes of lymphocytes.
Certain patterns are very strongly associated with various lymphoid malignancies. However, unlike IHC, which allows evaluation of phenotype in conjunction with tissue architecture, flow does not allow assessment of tissue architecture or cell morphology unless biopsy or cytology are also performed, thus flow results should not be interpreted in a vacuum. For proper interpretation, flow results must be evaluated in conjunction with other clinical variables, including presentation, history, and morphologic assessment.
Is all PARR testing equivalent?
No. Different labs analyze different types of specimens, use different primer sets, and use different detection technologies with differing sensitivities. Thus, PARR results may occasionally differ between labs. Not all labs have the ability to perform PARR analysis on formalin-fixed paraffin-embedded biopsy tissue and not all labs include review by a pathologist. VDx does!
At VDx, we work closely with Dr. Peter Moore and the Leukocyte Biology Laboratory at UC Davis and utilize an advanced set of primers that allow increased sensitivity over earlier published reports. Our primer set is continually undergoing refinement, improving the sensitivity and specificity of the assay.
What are the sensitivity and specificity of PARR?
Dog
T cell: ~95% sensitivity.
B cell: ~80% sensitivity.
Cat
T cell: >90% sensitivity.
B cell: ~50-60% sensitivity
The specificity of this test is excellent, especially when performed with rigorous quality control (all assays at VDx are performed in duplicate or triplicate) and in conjunction with morphologic assessment, IHC/ICC, and pathologist review.
Are there false negatives and false positives with PARR?
Yes. No lab test is perfect. False-negative results may occur for one or more of the following reasons:
- Clonal rearrangements that fall outside of the areas assessed by primers. The canine and feline genomic structure is complex and VDJ recombinations may occur at numerous sites, not all of which are detected by current primer sets.
- Mutations or deletions at the primer binding sites, which block primer binding and prevent amplification. This is particularly common with B cell lymphomas, due to the fact that B cells undergo somatic hypermutation as part of the affinity maturation of the immune response (and the reason that the sensitivity of the B cell assay is lower).
- Large numbers of reactive lymphocytes in a specimen, which may drown out the presence of a subtle neoplastic population. This is a particular risk in lymphoid tissues (lymph nodes, spleen, tonsil, GALT, etc…), processes (eg lymphoma), and cases of early emerging lymphoma where there is a heavy background of reactive or preneoplastic disease (eg early lymphoma arising out of IBD).
False positive results may occur for one or more of the following reasons:
- Rarely, very highly focused immunologic responses may present with a clonal pattern, ie “reactive clonal expansion”. Eg, lymphocyte clonality has been observed with E. canis infection, with idiosyncratic drug reactions, and in the T cell in regressing histiocytomas (PF Moore, pers comm).
- Very low numbers of B or T lymphocytes in a specimen.
- Additionally, while clonality patterns correlate strongly with lineage (clonal IgH rearrangements are generally associated with B cell neoplasia, TCRy with T cell neoplasia), some lymphomas may exhibit rearrangement of both IgH and TCRy (aka “cross-lineage rearrangement), complicating diagnosis. Thus, PARR can lead to erroneous results if used as a sole means of lineage determination.
These are reasons why morphology (path review) and IHC/ICC are critical parts of a complete workup. When the full workup is employed, the risk of false positives is very low.
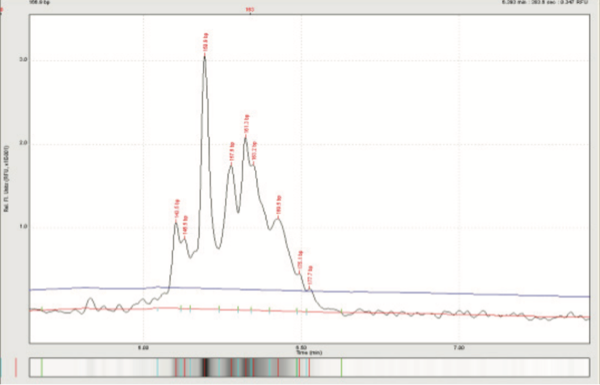
Feline TCRy assay: Clonal spikes among a polyclonal background = lymphoma among a background of / IBD.
Flow Cytometry
CANINE ONLY
What is Flow Cytometry and how can it help you manage cases?
Flow cytometry is an advanced technique for diagnosis and classification of hematopoietic neoplasia (leukemia and lymphoma). Because this is a heterogeneous group of diseases with a range of clinical presentations and progression, accurate classification of hematopoietic neoplasia is critical in predicting biologic behavior and selecting appropriate therapy. Flow cytometry, which is performed on live cells in liquid suspension (blood, bone marrow aspirates, and tissue aspirates in saline), allows identification of cell associated proteins that distinguish neoplastic cell populations by lineage (e.g. lymphoid vs. myeloid, B cell vs. T cell) and aberrant protein expression, allowing more precise classification for better prognostication and tailored therapy.
What are the advantages of Flow Cytometry?
Flow cytometry has advantages over traditional immunohistochemistry in that multiple cell proteins can be assessed simultaneously, sample collection is minimally invasive, and results are rapidly available. These features provide rapid and relevant prognostic information, and allow you to make timely, well-informed, decisions regarding clinical management of your patients.
Results are typically available the same business day (Monday – Friday) as sample receipt at the laboratory.
Getting Started
Sending a sample to VDx Veterinary Diagnostics is easy. Please call 1-530-753-4285 or email info@vdxpathology.com and a laboratory assistant will be more than happy to provide you with submission information and pricing.
Flow Cytometry Frequently Asked Questions
How should I prepare samples for flow cytometry?
How should I ship my sample to VDx for flow cytometry?
What samples are good candidates for immunophenotyping by flow cytometry?
How can Flow Cytometry be integrated into your current work up of hematopoietic neoplasia in dogs?
What are the limitations of Flow Cytometry?
What is Flow Cytometry and how can it help you manage cases?
What are the advantages of Flow Cytometry?
What is the turnaround time for flow cytometry results?
What is Flow Cytometry and how can it help you manage cases?
Flow cytometry is an advanced technique for immunophenotyping hematopoietic neoplasia (leukemia and lymphoma). Because this is a heterogeneous group of diseases with a range of clinical presentations and progression, accurate classification of hematopoietic neoplasia is critical in predicting biologic behavior and selecting appropriate therapy. Flow cytometry, which is performed on live cells in liquid suspension (blood, bone marrow aspirates, and tissue aspirates in saline), allows for identification of cell associated proteins that distinguish neoplastic cell populations by lineage (e.g. lymphoid vs. myeloid, B cell vs. T cell) and aberrant protein expression, allowing more precise classification for better prognostication and tailored therapy.
What are the advantages of Flow Cytometry?
Flow cytometry has advantages over traditional immunohistochemistry in that multiple cell associated proteins can be assessed simultaneously, sample collection is minimally invasive, and results are rapidly available. This information will allow you to make timely, well-informed, decisions regarding clinical management of your patients.
How can Flow Cytometry be integrated into your current work up of hematopoietic neoplasia in dogs?
Flow cytometry is typically recommended to guide clinical decision-making after a diagnosis of lymphoma or leukemia has already been established. Accurate classification of hematopoietic neoplasia requires assessment of disease distribution, cell morphology, and relevant clinicopathologic findings, in addition to immunophenotyping. As such, flow cytometry should always be interpreted in the context of clinical findings and concurrent cytologic or histologic evaluation of the affected tissue(s). In order to provide the best interpretation of flow cytometry results we recommend concurrent submission of air-dried, unstained blood smears or fine needle aspirate smears for cytologic evaluation (or copy of cytology/CBC report if evaluated elsewhere), along with your submission for flow cytometry testing.
What are the limitations of Flow Cytometry?
Flow cytometry can only be run on live cells. Samples must be shipped expediently so that they can be processed, ideally, within 24 hours of sample collection. See FAQs for recommendations regarding sample collection and shipping. It can be difficult to interpret heterogeneous samples and samples with small numbers of neoplastic cells. Neoplastic cells can sometimes have unexpected atypical protein expression that precludes definitive lineage classification. In these cases, additional tests, such as PCR for Antigen Receptor Rearrangement (PARR), may help to further characterize these cells.
What samples are good candidates for immunophenotyping by flow cytometry?
- Peripheral blood with a lymphocytosis >10,000 cells/microliter.
- Peripheral blood with atypical cells.
- If very few atypical cells are present flow cytometry may be inconclusive.
- Lymph node, bone marrow, or other tissue aspirate from a patient with a defined population of atypical cells and/ or previous diagnosis of lymphoma or leukemia.
How should I prepare samples for flow cytometry?
REFRIGERATE (do not freeze) AFTER COLLECTION
- For Whole Blood
- Use an EDTA (purple top) tube
- Add a minimum of 1.0 ml of blood
OR
- For Tissue Aspirate
- Use a white top tube (do not use serum separator tubes or tubes with other additives)
- Add 1ml of sterile 0.9% saline
- Add 0.1 ml of canine serum (from the patient or another donor) to aid in cellular preservation, if available
- Add a minimum of three fine needle aspirates
- To improve the yield of tissue aspirates we recommend using gentle negative pressure during collection
- Excessive negative pressure applied during sample collection can lyse cells rendering the sample unsuitable for flow cytometry analysis
- An ideal sample will look cloudy and have minimal blood contamination
- A small amount of blood in the sample is okay but heavy blood contamination should be avoided
Flow cytometry analysis can only be performed on samples containing >60% viable cells.
How should I ship my sample to VDx for flow cytometry?
DO NOT FREEZE
- Samples should be refrigerated after collection and kept cool on ice** during shipping
- **Avoid direct contact of the sample with ice packs – wrap fluid tubes in a paper towel and place in a sealable plastic bag
- **Avoid direct contact of the sample with ice packs – wrap fluid tubes in a paper towel and place in a sealable plastic bag
- Blood smears and air-dried tissue aspirate smears should be shipped in appropriate containers to prevent slide breakage and minimize exposure to moisture
- Do not refrigerate smears
- Do not package smears with biopsy samples (exposure to formalin vapors will degrade cytologic preparations)
- Northern California Courier Clients Collect and store samples as described above. Keep tube refrigerated prior to pick up
- FedEx/UPS Clients Samples should be shipped via FedEx Priority Overnight/UPS Next Day Air the same day of collection. Please do not ship samples on Friday for Saturday or Monday delivery
Ship samples toVDx Veterinary Diagnostics
215 C Street, Suite 301
Davis, CA, 95616
What is the turnaround time for flow cytometry results?
Results are typically available next business day (Monday – Friday) from sample pick-up or receipt in the laboratory via FedEx/UPS.
